Interest in developing batteries based on sodium has recently spiked because of concerns over the sustainability of lithium, which is found in most laptop and electric vehicle batteries.

Developed in the 1980s and recognized by the 2019 Nobel Prize in Chemistry, the lithium-ion battery has become one of the most commonly used batteries in the world. It powers most phones and laptops, and it has driven the surge in electric vehicle production. Like most batteries, a lithium-ion battery consists of three main components: a positive electrode (cathode), a negative electrode (anode), and an ion-transporting medium (electrolyte) in between the two. There are various choices for the materials used for each component, but the most common design has an anode made of graphite (carbon); a cathode made of a lithium-containing metal oxide, such as lithium cobalt oxide or lithium manganese oxide; and an electrolyte that combines a lithium-based salt and an organic solvent.
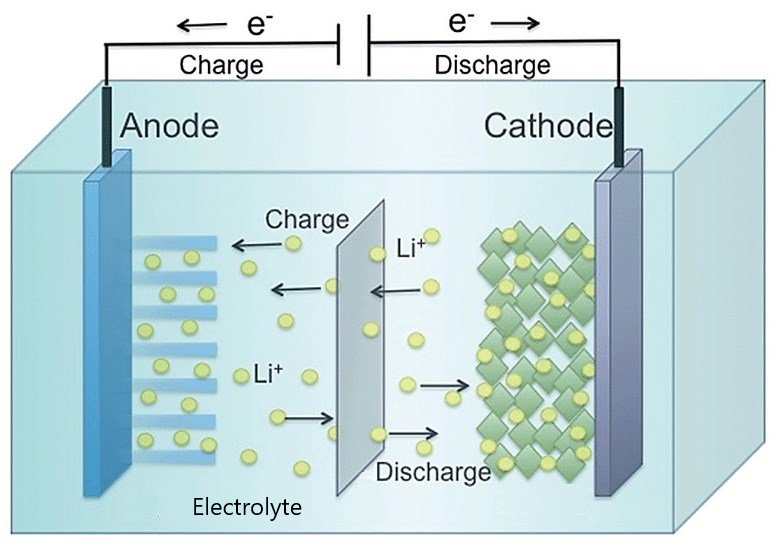
A lithium-ion battery consists of an anode, a cathode, and a liquid electrolyte between them. Lithium ions move toward the anode when the battery charges and then move back to the cathode when it discharges. Electric current flows into and out of the battery through the wire connections at the two electrodes.
When the battery is working (discharging), lithium ions come out of the anode and move through the electrolyte to the cathode where they are absorbed. When the lithium ions enter the cathode, a chemical reaction occurs that essentially “draws” electrons into the cathode from the connecting wire. During charging, electrons flow out of the cathode, freeing the lithium ions so that they flow back into the anode.
Lithium-ion batteries have a number of attractive attributes. First and foremost, they are rechargeable and have a high-energy density of 100–300 watt hours per kilogram (Wh/kg), compared to 30–40 Wh/kg for common lead-acid batteries. That high density means your laptop or cellphone can have a battery that lasts throughout the day without weighing you down. In the case of electric vehicles, a typical battery can weigh around 250 kg and supply around 50,000 Wh of energy, which is typically enough to drive 200 miles (320 km). Many environmentalists see this capability as our ticket for transitioning away from fossil fuels.
However, not everything about lithium-ion batteries is an environmentalist’s dream. The main issue involves the materials, since the extraction of lithium is resource intensive, and the mining of some of the metal ingredients is polluting. There is also a lack of recycling infrastructure for today’s lithium-ion batteries, Meng says. “The carbon footprint and the sustainability of the current way of making lithium-ion batteries is less than ideal.”
In addition to environmental concerns, the battery market is highly volatile, in part because the world has a limited number of lithium-rich regions. During the COVID pandemic, for example, the supply chain was cut off, and the price of lithium shot up. There are similar concerns over other lithium-ion-battery materials, such as nickel, copper, and graphite, which are also limited resources.
Lithium-ion alternatives include solid-state batteries (in which the liquid electrolyte is replaced by a solid one) and magnesium-ion batteries (in which magnesium ions replace lithium ions). Most of these options are still under development. And some of them also have issues concerning the availability of resources.
By contrast, sodium is abundant in seawater (although a more usable source is sodium ash deposits, which can be found in many regions of the world). And because sodium shares so much chemistry with lithium, sodium-ion batteries have been developing quickly and are already being commercialized.
However, sodium and lithium atoms have differences, two of which are relevant for battery performance. The first difference is in the so-called redox potential, which characterizes the tendency for an atom or molecule to gain or lose electrons in a chemical reaction. The redox potential of sodium is 2.71 V, about 10% lower than that of lithium, which means sodium-ion batteries supply less energy—for each ion that arrives in the cathode—than lithium-ion batteries. The second difference is that the mass of sodium is 3 times that of lithium.
Together these differences result in an energy density for sodium-ion batteries that is at least 30% lower than that of lithium-ion batteries. When considering electric vehicle applications, this lower energy density means that a person can’t drive as far with a sodium-ion battery as with a similarly sized lithium-ion battery. In terms of this driving range, “sodium can’t beat lithium,” Tarascon says.
The energy density is also a problem when considering the overall environmental impact of a battery. Weil and his colleagues performed a comparison of sodium-ion batteries to lithium-ion batteries, looking at a number of environmental factors such as greenhouse gas emissions and resource usage. Although sodium-ion batteries do not require as many of our planet’s limited resources, they currently release more greenhouse gases during production than an equivalent energy’s worth of lithium-ion batteries. The reason is that larger quantities of materials need to be processed into batteries to produce the same amount of energy.
Weil says that this report provides a current snapshot, and in time, the environmental impact of sodium-ion batteries will likely improve. “We are convinced that they could have an even better overall performance than present lithium-based systems,” he says.

A comparison of lithium-ion and sodium-ion batteries. From left to right the columns show abundance of lithium and sodium in Earth’s crust (in parts per million), energy density (in watt hours per kilogram), battery lifetime (in number of charging cycles), greenhouse gas emissions from battery production (in equivalent kilograms of carbon dioxide emissions), and resource usage (in equivalent grams of the element antimony, based on a calculation that accounts for all of the abundances of the batteries’ materials). Values apply to certain battery designs and may not be correct for every battery.
There are other differences between the two elements, some of which work in sodium’s favor. For example, sodium ions can travel faster through the battery materials than lithium ions, which might seem counterintuitive, given that sodium is heavier. Tarascon explains that a sodium ion has a diffuse electron cloud that allows it to slip between atoms more easily than a lithium ion, with its highly concentrated charge. The faster motion of a sodium ion can lead to higher power and faster charging in sodium-ion batteries.








