A Lithium-ion battery is the storehouse of energy. To harness the enormous amount of solar, wind, tidal energy – batteries are the only option available to us.
Advancement in battery tech has enabled the development of newer methods to store energy. The promise of clean and noiseless energy makes this field our first-step into the future.
Lithium is the lightest material known to us, with density (0.534 g/cm3), it is almost half as dense as water (1 g/cm3). It belongs to Group 1 (called Alkali) of the periodic table with one free electron in their outer shell which makes them highly reactive.
This metal is abundant in the Earth’s surface and can be mined and hence is cheap, making it an ideal choice for batteries.
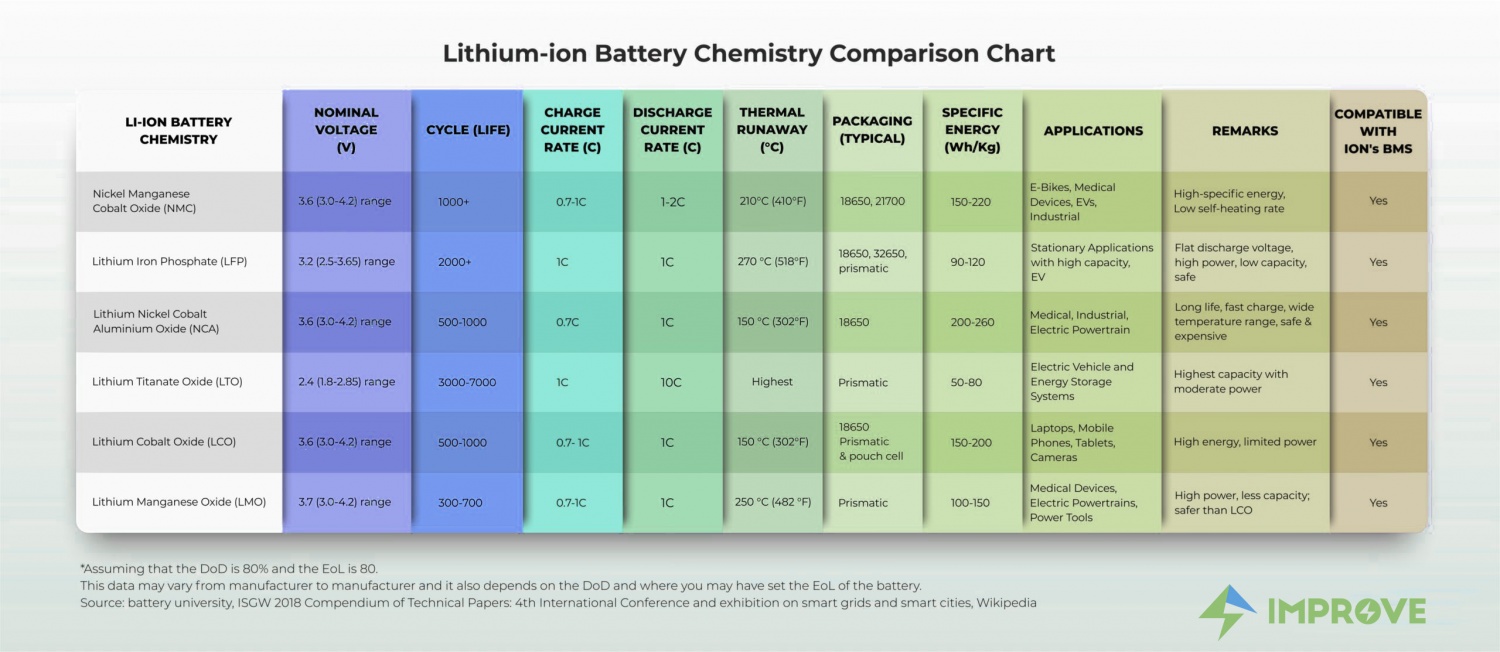
There are a few specific chemistries, within the broad Lithium-ion battery category that possess unique advantages that helps battery pack makers select the ideal Lithium-ion battery chemistry for various applications.
Important terms specific to Lithium-ion battery chemistry:
- Nominal Voltage: It is the system voltage at which the device is designed to operate. The rated voltage is usually higher than the nominal voltage to ensure the safe operation of the device.
- Cycle: The charge and discharge make a Cycle
- The current rate (C): This is used to determine the fast charging capability of a battery.
- Thermal Runaway: It is a condition where an increase in temperature changes the conditions which increase the temperature further, often leading to disaster.
- Specific Energy: It is the amount of energy stored per unit mass (Wh/Kg).
Most common types of Lithium-ion battery chemistries:
Click on the image to enlarge.
1. Lithium Cobalt Oxide (LiCoO2) — LCO
It has high specific energy but low specific power which means that charging and discharging with Current Rate (C) above rated values shorten the battery’s life. It has a cobalt Oxide Cathode and Graphite anode. The cathode is layered.
During discharge, the Li-ions move from anode to cathode. The flow reverses during charging. The graphite anode limits its cycle life hence it has a short life span. It has low thermal stability. Its main applications are mobile phones, laptops, cameras, etc.
2. Lithium Manganese Oxide (LiMn2O4) — LMO
The Manganese Oxide forms a 3D-spinel structure that improves the ion flow on the electrode. This results in lower internal resistance, which enables fast charging and high-current discharging. Adding to these, spinel structure also packs extra thermal stability. Making it safer compared to LCO. The LMO has a lower cycle life.
Most Li-manganese batteries blend with lithium nickel manganese cobalt oxide (NMC) to enhance the precise energy and prolong battery life. These three active metals, as well as silicon enhancement, can enhance specific energy (capacity), specific power (load capability) or longevity.
Li-manganese has high power and less capacity. It is used for power tools, medical devices as well as in electric vehicle powertrain.
3. Lithium Nickel Manganese Cobalt Oxide (LiNiMnCoO2) — NMC
The cathodic combination of Nickel Manganese Cobalt is successful as it can be tailored to be either Energy cells or Power Cells. NMC has a good performance and excels in specific energy. This Lithium-ion battery can deliver a continuous discharge current.
The advantage of Nickel is its high specific energy but it has poor stability. Whereas Manganese achieves low internal resistance by forming a spinal structure. Unlike Nickel, Manganese offers low specific energy.
Their combination enhances each other’s performances. New electrolytes can boost capacity. It has the lowest self-heating rate and improved cycle life. This is a battery of choice for electric powertrains and industrial applications.
4. Lithium Iron Phosphate (LiFePO4) — LFP
In this type, Li- Phosphate is used as the cathode since it has good electrochemical performance with low resistance. This is because of the nano-scale phosphate cathode material.
Various advantages of this Lithium-ion battery chemistry include – long lifecycle, good thermal stability, high current rating, enhanced safety, and tolerance in case of abuse.
Li-Phosphate is often used to replace lead-acid batteries as it has a moderate specific energy and has a very low tolerance for moisture, which reduces its cycle life drastically.
Other characteristics include a very flat voltage discharge curve but low capacity. LFP is one of the safest Li-ions in the market.
5. Lithium Nickel Cobalt Aluminum Oxide (LiNiCoAlO2) — NCA
NCA batteries are gaining importance in electric powertrains and grid storage. They are not common in the consumer industry but are promising for the automotive Sector. Tesla, the US-based EV manufacturer researches this technology and uses this Lithium-ion battery chemistry.
NCA has the highest Specific Energy, good Specific Power, and a long life span. High cost and marginal safety are the negatives.
6. Lithium Titanate (Li2TiO3) — LTO
In this technology, Li-titanate replaces the graphite in the anode of a typical Lithium-ion battery and the material forms into a spinel structure.
The cathode can be lithium manganese oxide or NMC. Li-titanate can be fast-charged and delivers a high discharge current 10 times the rated capacity. The cycle count is higher than the regular Li-ion batteries.
Li-titanate excels in safety and has excellent low-temperature discharge characteristics and life span. The applications this lithium ion battery chemistry power include electric powertrains, UPS and solar-powered street lighting.